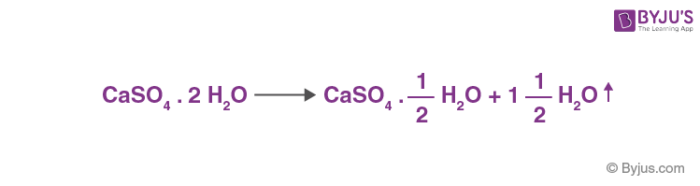
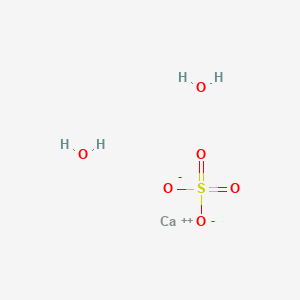
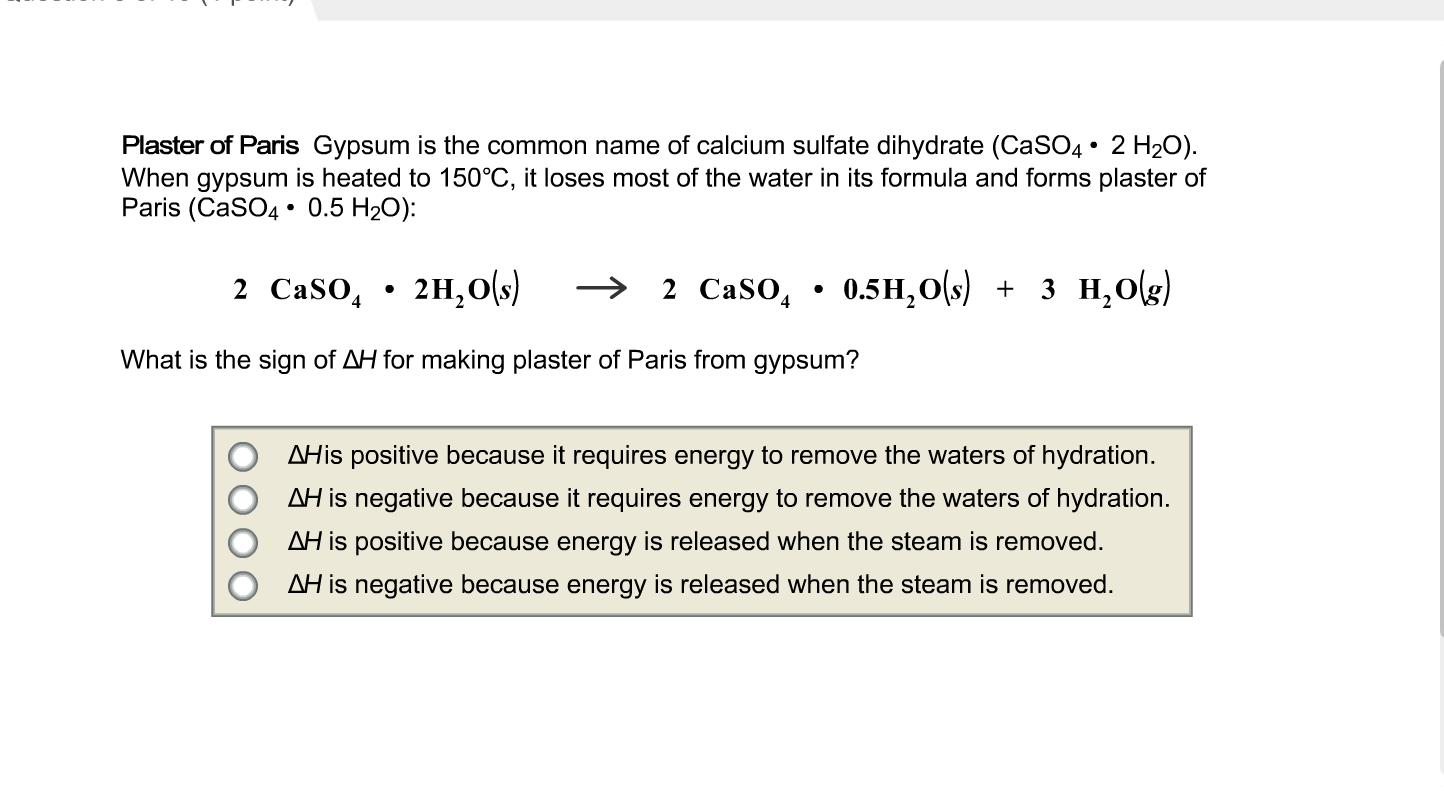
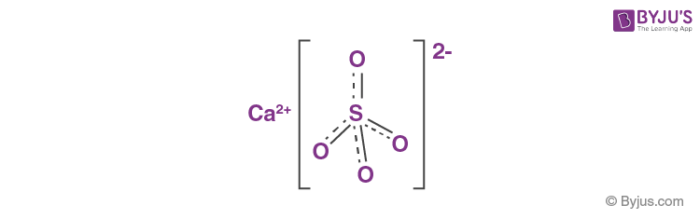
Influence of the Process Parameters on the Formation of CaSO4·0.5H2O Whiskers – topic of research paper in Nano-technology. Download scholarly article PDF and read for free on CyberLeninka open science hub.

Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Journal of Chemical & Engineering Data

Crystals | Free Full-Text | Influence of Alkyl Trimethyl Ammonium Bromides on Hydrothermal Formation of α-CaSO4·0.5H2O Whiskers with High Aspect Ratios

Direct synthesis of single-phase α-CaSO4·0.5H2O whiskers from waste nitrate solution - ScienceDirect
Force field for calcium sulfate minerals to predict structural, hydration, and interfacial properties

Formation and Transformation of Five Different Phases in the CaSO4−H2O System: Crystal Structure of the Subhydrate β-CaSO4·0.5H2O and Soluble Anhydrite CaSO4 | Chemistry of Materials
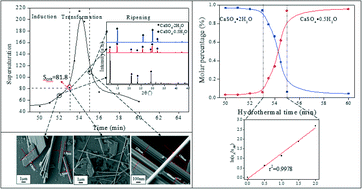
Supersaturation-induced hydrothermal formation of α-CaSO4·0.5H2O whiskers - CrystEngComm (RSC Publishing)
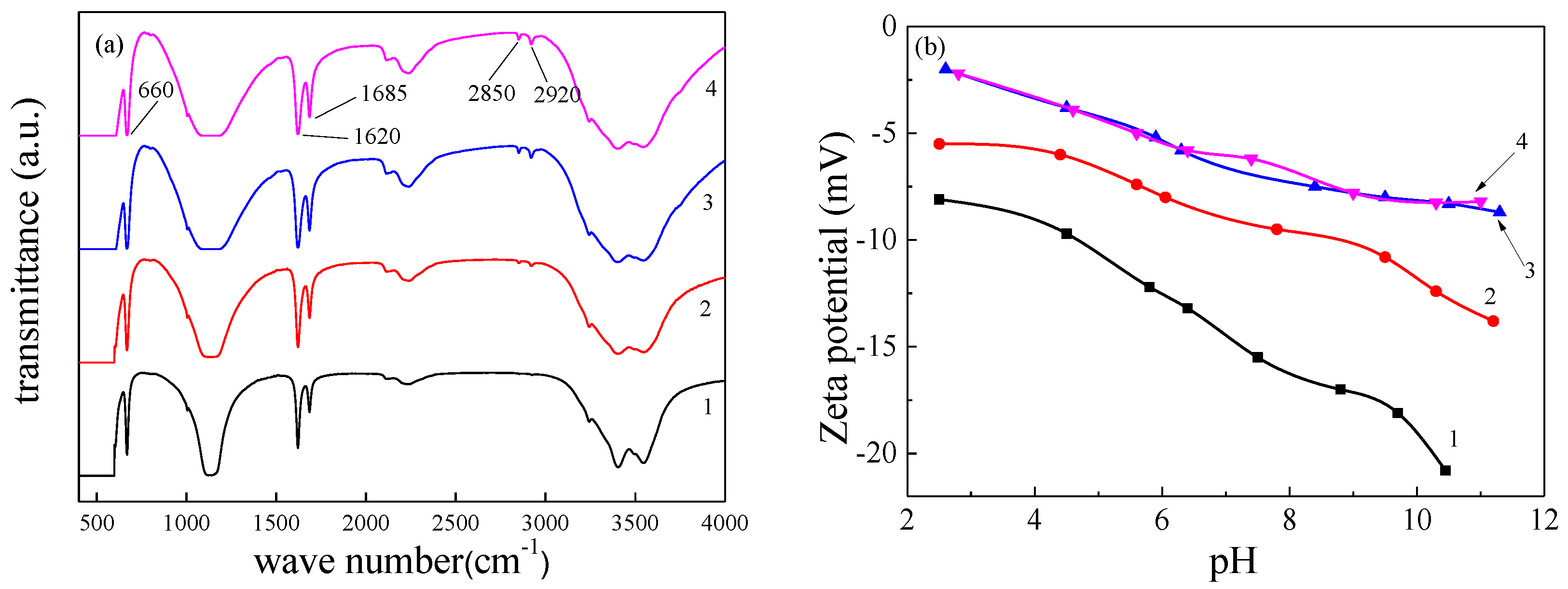
Crystals | Free Full-Text | Influence of Alkyl Trimethyl Ammonium Bromides on Hydrothermal Formation of α-CaSO4·0.5H2O Whiskers with High Aspect Ratios

PDF) Thermal behaviour and kinetics of dehydration in air of bassanite, calcium sulphate hemihydrate (CaSO4· 0.5 H2O), from X-ray powder diffraction | Paolo Ballirano - Academia.edu